Our group uses computational and analytical tools to understand the behavior and properties of soft matter systems and in particular macromolecular physics. A complete index of our publications may be found here.
One of the common features of different research areas listed below is reversible interactions, such as hydrogen bonding, donor-acceptor, ligand-receptor interactions, which can be broken and reformed providing a self-healing (i.e. self-adjusting to external conditions) property, which is common among biological systems and is essential for smart material design. Among areas of our current research are the following:
Macromolecular hydration, hydrogen bonding, polyethylene oxide
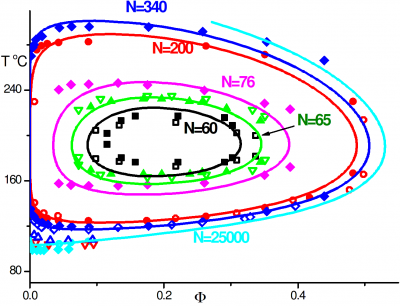
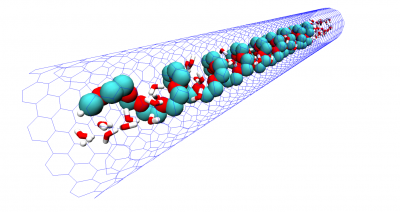 Macromolecular interaction with water is one of the fundamental, yet not so well understood, interactions determining chain conformation and often other properties. Understanding the hydration of polymers is essential for development of new biomaterials and optimization of their performance and can also provide some insights on biopolymer behavior as well. We investigate of the mechanism of polymer hydration and hydrogen bonding in particular and analyze factors influencing it. In our earlier work we have shown that hydrogen bonding is responsible for closed-loop phase behaviour in hydrogen bonded polymer blends and aqueous solutions of polyethylene oxide (PEO). In our recent work, we show that stability of the hydration shell of polyethylene oxide under confinement in a carbon nanotube is responsible for stabilization of the helical structure, which is not observed in solution. Similarly we demonstrate that stable hydrogen bonding with isobutyric acid in pure or mixed aqueous solutions results in helical conformation of PEO in agreement with experimental results. Understanding hydration of polyethylene oxide grafted on planar and curved surfaces or being part of self-assembled diblock copolymer micelles can be of particular importance in biomedical applications where PEO is routinely used to minimize protein adsorption.
Macromolecular interaction with water is one of the fundamental, yet not so well understood, interactions determining chain conformation and often other properties. Understanding the hydration of polymers is essential for development of new biomaterials and optimization of their performance and can also provide some insights on biopolymer behavior as well. We investigate of the mechanism of polymer hydration and hydrogen bonding in particular and analyze factors influencing it. In our earlier work we have shown that hydrogen bonding is responsible for closed-loop phase behaviour in hydrogen bonded polymer blends and aqueous solutions of polyethylene oxide (PEO). In our recent work, we show that stability of the hydration shell of polyethylene oxide under confinement in a carbon nanotube is responsible for stabilization of the helical structure, which is not observed in solution. Similarly we demonstrate that stable hydrogen bonding with isobutyric acid in pure or mixed aqueous solutions results in helical conformation of PEO in agreement with experimental results. Understanding hydration of polyethylene oxide grafted on planar and curved surfaces or being part of self-assembled diblock copolymer micelles can be of particular importance in biomedical applications where PEO is routinely used to minimize protein adsorption.
Biomedical applications of nanoparticles, nanoparticle targeting
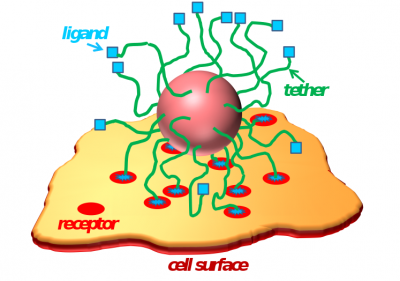
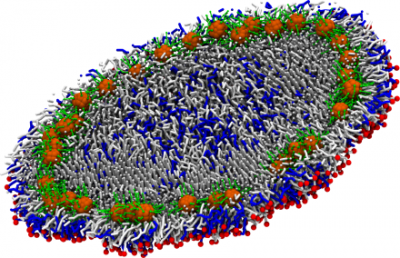 Progress in nanobiotechnology has made available a range of nanoparticles (diblock copolymer micelles, polymer modified gold nanoparticles, dendrimers, liposomes, etc.) for diagnostic and therapeutic applications. Our research in this area has been focused on understanding the strength and selectivity of interactions between a polymer-tethered nanoparticle (or a polymer layer) functionalized by ligands and a cell surface containing receptors. We have also studied drug-polymer interactions and drug release from polymer micelles. Besides polymer-based nanoparticles, more recently we also studied encapsulation of gold nanoparticles (AuNPs) modified by hydrophobic tethers into lipid nanodiscs. Our course-grained simulations predict formation of a gold ring along the circumference of the nanodisc, which can stall vesiculation process at an “open vase” state. Experimental studies from our collaborator’s group (Prof. Mu-Ping Nieh) show interesting luminescent properties of these AuNP/lipid assemblies which can potentially be employed in nanobiomedicine.
Progress in nanobiotechnology has made available a range of nanoparticles (diblock copolymer micelles, polymer modified gold nanoparticles, dendrimers, liposomes, etc.) for diagnostic and therapeutic applications. Our research in this area has been focused on understanding the strength and selectivity of interactions between a polymer-tethered nanoparticle (or a polymer layer) functionalized by ligands and a cell surface containing receptors. We have also studied drug-polymer interactions and drug release from polymer micelles. Besides polymer-based nanoparticles, more recently we also studied encapsulation of gold nanoparticles (AuNPs) modified by hydrophobic tethers into lipid nanodiscs. Our course-grained simulations predict formation of a gold ring along the circumference of the nanodisc, which can stall vesiculation process at an “open vase” state. Experimental studies from our collaborator’s group (Prof. Mu-Ping Nieh) show interesting luminescent properties of these AuNP/lipid assemblies which can potentially be employed in nanobiomedicine.
Thermodynamics and Kinetics of self-assembly
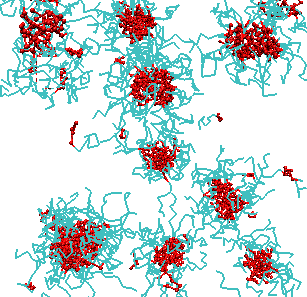
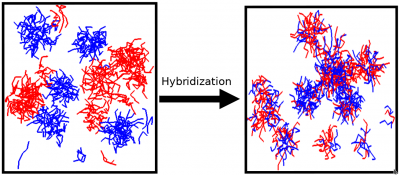 Spontaneous self-assembly is one of the fundamental phenomena in macromolecular systems, both synthetic and biopolymer. The driving force for self-assembly is the tendency of the system to minimize undesirable interactions, such as hydrophobic-hydrophilic interactions and/or maximize favorable interactions, such as donor-acceptor interactions. As a result of self-assembly a variety of complex nanostructures can be formed. Understanding the fundamental principles of self-assembly is essential for development of nanotechnological applications of soft matter systems and is the key to understanding the behavior and functions of biopolymers. We studied the self-assembly with micelle formation in different block-copolymer systems. Predictions were made regarding the equilibrium structure and the kinetic mechanisms of self-assembly. We have demonstrated that the hydrophobicity, block length and chain architecture all contribute to the kinetics of chain exchange between micelles at equilibrium. Our recent results indicate that there is a co-influence of different block copolymers on chain exchange kinetics at equilibrium.
Spontaneous self-assembly is one of the fundamental phenomena in macromolecular systems, both synthetic and biopolymer. The driving force for self-assembly is the tendency of the system to minimize undesirable interactions, such as hydrophobic-hydrophilic interactions and/or maximize favorable interactions, such as donor-acceptor interactions. As a result of self-assembly a variety of complex nanostructures can be formed. Understanding the fundamental principles of self-assembly is essential for development of nanotechnological applications of soft matter systems and is the key to understanding the behavior and functions of biopolymers. We studied the self-assembly with micelle formation in different block-copolymer systems. Predictions were made regarding the equilibrium structure and the kinetic mechanisms of self-assembly. We have demonstrated that the hydrophobicity, block length and chain architecture all contribute to the kinetics of chain exchange between micelles at equilibrium. Our recent results indicate that there is a co-influence of different block copolymers on chain exchange kinetics at equilibrium.
Associating and supramolecular polymers and networks
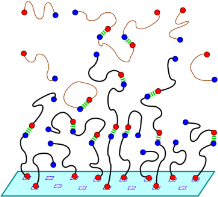
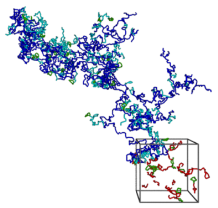 Reversibly associating polymers are a class of materials which employ reversible interactions, such as hydrogen bonding, donor-acceptor or other binary reversible interactions. Reversible associating polymers are responsive to external stimuli to a much greater extent than classical polymers and possess self-healing properties, which make them attractive in practical applications.Our group was the first to study by means of computer modelling and analytical theory the self assembly and properties of supramolecular polymers with a head-to-tail associating motif. We have shown that both the rigidity of the spacers and orientational specificity of the association are important for ring-chain equilibrium in these supramolecular systems. Both the thermodynamics and rheology of associating polymer solutions were studied. We also investigated self-assembly of head-to tail associating polymers on planar surfaces and analyzed gelation of metallo-supramolecular polymers capable of 3:1 ligand-metal complexation. Predictions have been made regarding the required condition for network formation and properties of the resultant network have been characterized.
Reversibly associating polymers are a class of materials which employ reversible interactions, such as hydrogen bonding, donor-acceptor or other binary reversible interactions. Reversible associating polymers are responsive to external stimuli to a much greater extent than classical polymers and possess self-healing properties, which make them attractive in practical applications.Our group was the first to study by means of computer modelling and analytical theory the self assembly and properties of supramolecular polymers with a head-to-tail associating motif. We have shown that both the rigidity of the spacers and orientational specificity of the association are important for ring-chain equilibrium in these supramolecular systems. Both the thermodynamics and rheology of associating polymer solutions were studied. We also investigated self-assembly of head-to tail associating polymers on planar surfaces and analyzed gelation of metallo-supramolecular polymers capable of 3:1 ligand-metal complexation. Predictions have been made regarding the required condition for network formation and properties of the resultant network have been characterized.